The virotype PRRSV EU Vax RT-PCR Kit from INDICAL is the first assay to simultaneously detect and differentiate all major live-attenuated PRRSV-1 (EU genotype) vaccines. PRRSV-1 predominates in Europe but also occurs in Asia and America.
Save Time and Reduce Costs
With the virotype PRRSV EU Vax RT-PCR Kit you can:
- Detect all major PRRSV-1 vaccines in a single run
- Rapidly and cost-effectively differentiate vaccines compared to sequencing
- Distinguish infected from vaccinated animals (DIVA) when combined with the virotype PRRSV 2.0 RT-PCR Kit (VT282325)
High Performance and Easy of Use
- Kit combines two ready-to-use Triplex RT-qPCRs covering all major PRRSV-1 vaccines
- Exogenous Internal Controls to monitor PCR efficiency
- Highly sensitive — detects as few as 5 copies per sample
- Results in just 67 minutes*
- Compatible with most PCR thermocyclers
- Optimized for IndiMag/IndiSpin extraction workflows
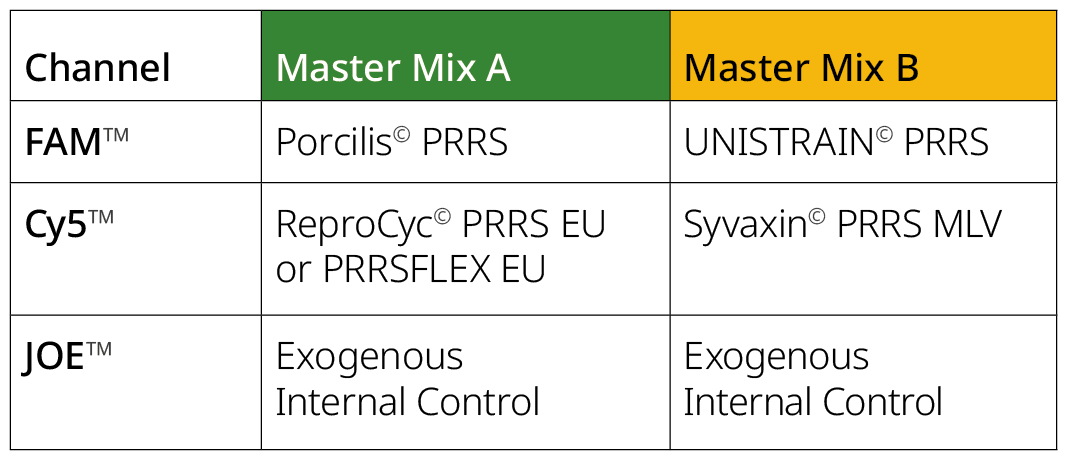
Figure 1: The PRRSV EU Vax RT-PCR Kit contains two ready to use Master Mixes, A and B, with the corresponding targets and detection channels detailed below:
Flexible with Multiple Validated Swine Sample Types
- Serum
- Plasma
- Saliva
- Tissue
- Processing fluids
Porcine Reproductive and Respiratory Syndrome Virus (PRRSV)
Infections with PRRSV in swine are highly prevalent and economically very important for the swine industry. PRRSV is an RNA virus that belongs to the order Nidovirales, family Arteriviridae. PRRS virus infection can cause respiratory disease in piglets and reproductive failure in pregnant sows.
Monitoring vaccination success requires differentiating vaccine from wild-type PPRSV strains. Sequencing can be costly, time-consuming and incompatible with weak positive samples. The virotype PRRSV EU Vax RT-PCR Kit offers a fast, practical alternative to confirm PRRSV-1 vaccine status, saving time in the lab while delivering reliable, actionable results for confident herd health decisions.
Currently, four commercial live-attenuated vaccines are used for PRRSV-1 control (see figure below):
*Agilent Mx3005P
For up-to-date licensing information and product-specific disclaimers, see the respective handbook or user manual. Assays for veterinary use only. Regulatory requirements vary by country, product may not be available in your geographic area. PCR and ELISA kits availability/distribution: Outside the U.S. and Canada.
| Pathogen type | Virus |
| Product type | Ready-to-use assay |
| Species | Swine |
| Pathogen investigated | Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) |
| Technology | RT-qPCR |
|
Handbooks
Handbuch virotype PRRSV EU Vax RT-PCR Kit (August 2025, DE)
|
|
Brochures
|
|
Handbooks
Handbook virotype PRRSV EU Vax RT-PCR Kit (August 2025, EN)
|
| Certificates of Analysis (CoA) |
Request a Certificate of Analysis and see the quality control data for your product. |
| Safety Data Sheets |
Safety Data Sheets (SDS) for product components are available on request. |